The Feske lab investigates how ion channels regulate immune responses in humans and mice. Channels and transporters facilitate the movement of charged ions across lipid membranes in all cells of the body. They are well recognized to control the function of excitable tissues such as brain and heart, but their function in immune cells is much less understood. It has long been known that stimulation of immune cells via antigen receptors results in Ca2+ influx from the extracellular space, and that the resulting Ca2+ signals are required for leukocyte function. Immunoreceptor-induced Ca2+ influx is mainly mediated by Ca2+ release-activated Ca2+ (CRAC) channels that are formed by ORAI and STIM family proteins. CRAC channels and their role in immunity are a major research focus of the Feske lab.
Besides Ca2+, other ions (including Mg2+, Zn2+, Na+, K+, Cl-) and the channels that conduct them have been shown to regulate immune responses. There are still many gaps in our mechanistic understanding of how ion channels regulate immune function. For instance, how do ions other than Ca2+ signal and control immune cell function? Which ion channels function in immune cells and how do they differ between the various types of lymphoid and myeloid immune cells? How do ion channels control complex immune responses to infection, tumors or autoimmunity? Can ion channels in immune cells be targeted with to treat autoimmune disease, allergies or cancer? We are trying to answer these questions by studying ion channels in humans and mice for their mechanistic and physiological functions in immunity.
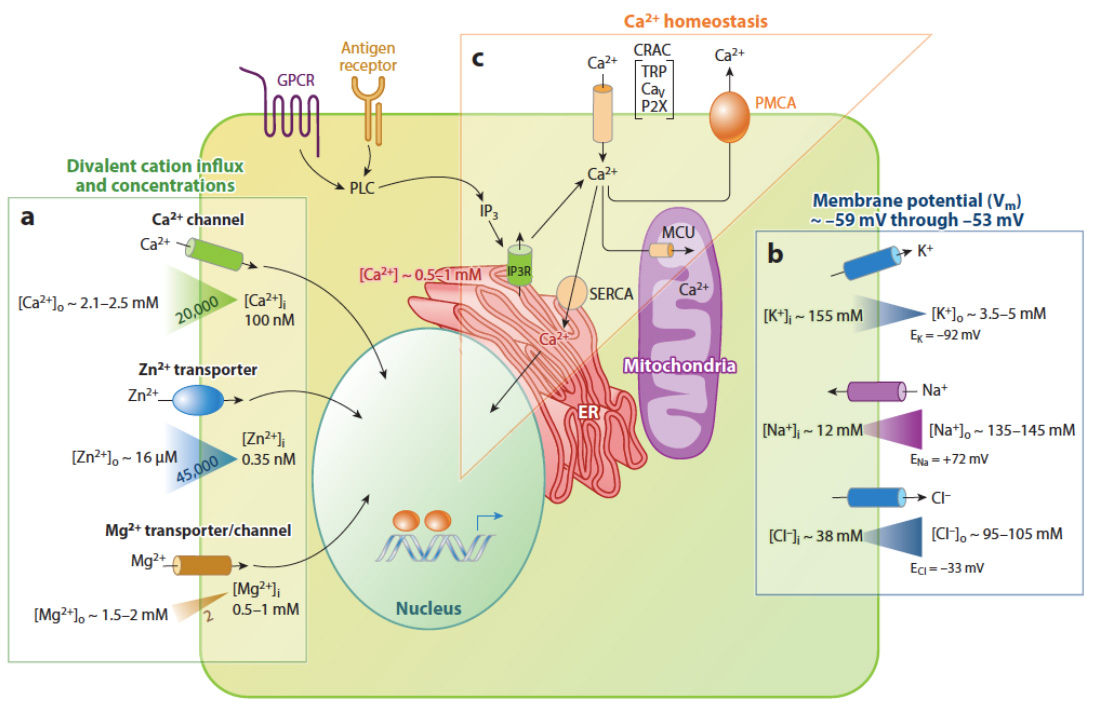
Ion concentrations and signaling in immune cells. A variety of ion channels and transporters control ion concentrations in the cytoplasm and intracellular organelles and thereby the function of immune cells. (a) Divalent cation channels and transporters and the concentrations of extra- and intracellular Ca2+, Mg2+, and Zn2+ ions (concentration gradients indicated by triangles). (b) Monovalent cation (K+, Na+) and anion (Cl−) channels that control the membrane potential (Vm) in immune cells. (c) Mechanisms regulating Ca2+ homeostasis in immune cells. Antigen receptor and GPCR binding results in PLC activation, production of IP3 and Ca2+ release from ER Ca2+ stores via IP3R channels resulting in a transient increase in [Ca2+]i followed by activation of store-operated Ca2+ entry (SOCE). The resulting increase in [Ca2+]i is balanced by Ca2+ reuptake into the ER via SERCA pumps, Ca2+ export via PMCA pumps, and uptake into the mitochondrial matrix via the mitochondrial Ca2+ uniporter (MCU). From: Feske et al. 2015 Ann Rev Imm 33:291-353.